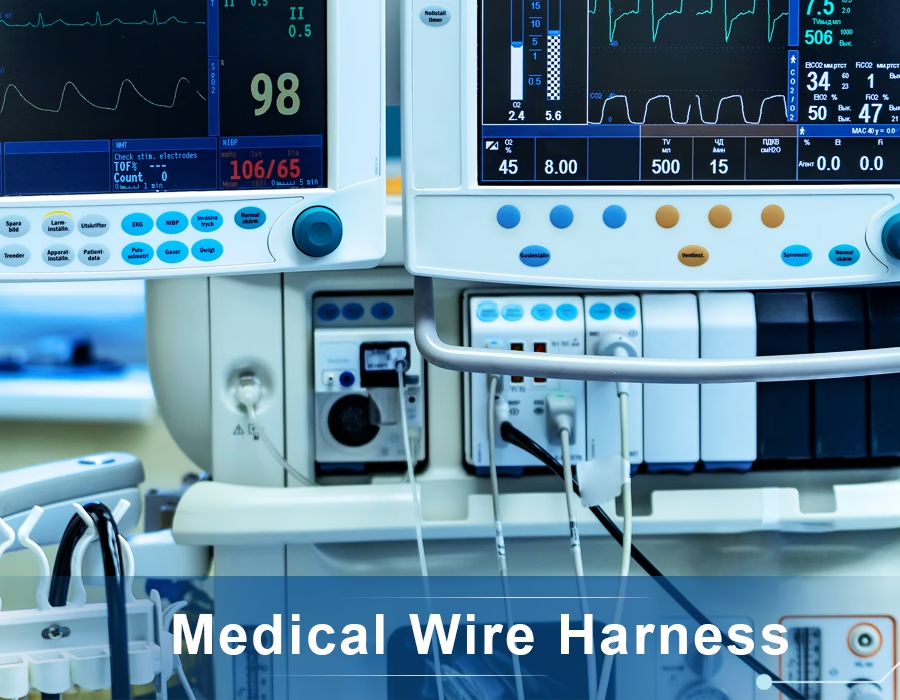
An operating room with interconnected medical gadgets emphasizes the significance of reliable cabling. Every sensor, monitor, and surgical tool in healthcare technology relies on proper wiring to function. A medical wire harness is a specialized assembly of wires, connections, and terminals that powers and connects the internal components of medical devices. Consider it the device’s nervous system, grouping dozens (or even hundreds) of wires into a single structured unit to ensure efficient signal and power flow. This precise design reduces loose connections and human wiring errors, which is essential when a patient’s life is at stake.
To meet stringent healthcare standards, medical wire harnesses use high-quality materials (copper conductors, PVC/silicone insulation, etc.) and specialist connectors. Harness components, for example, may be insulated to prevent electromagnetic interference (EMI) and made of medical-grade polymers that can be sterilized repeatedly.
A medical harness is often constructed with copper conductors (for optimal conductivity) covered with insulating fillers and signal-protecting jackets (such as PTFE or silicone). The outer layer is a waterproof, durable covering (often made of PVC or a similar material) that protects against moisture, heat, and abrasion. Custom labels and clips are frequently used to fasten the harness to equipment. Every component is carefully selected for its longevity and biocompatibility; in fact, many harnesses are engineered to exceed ISO 10993 material safety standards, as well as RoHS/REACH hazardous substance limits.
Typical Applications in Medical Equipment
Medical wire harnesses are included in nearly every type of medical device. They are specific to the device’s purpose and environment. Key applications include:
- Imaging Systems (MRI, CT, X-ray, ultrasound, endoscopes) – These machines use specialized harnesses (typically with coaxial cables) to transmit high-frequency data and power with minimal loss. For example, MRI and CT scanners employ protected wire assemblies to provide accurate image signals, but ultrasound devices use flexible harnesses for probe connections.
- Patient Monitoring (ECG/EKG, EEG, Vital Sign Monitors) – Harnesses deliver bioelectrical impulses from electrodes and sensors to monitoring equipment. In ECG equipment, a 10-lead cable assembly transmits heart signals precisely; in intensive care, multi-sensor harnesses keep ICU monitors running continually.
- Surgical and Therapeutic Devices – Custom harnesses are used to control and power robotic surgery arms, electrosurgical generators, and other OR equipment. Ventilators, infusion pumps, and dialysis machines also rely on long-lasting wiring harnesses that can withstand continuous use and cleaning.
- Laboratory and Diagnostic Instruments – Harnesses connect sensors, motors, and controllers on automated analyzers and lab robots. Cable assemblies are used to ensure correct signal transmission in blood analyzers, point-of-care test devices, and even handheld glucose meters.
- Wearable and Home-Health Devices – Wearable monitoring and home therapy equipment include flexible, lightweight straps. These assemblies must strike a compromise between durability and patient comfort, which is frequently achieved with silicone jackets and snap-on connectors.
In summary, you’ll find medical harnesses inside practically every diagnostic, imaging, monitoring, or therapeutic machine – from MRI coils and ultrasound probes to ICU monitors and surgical robots. They enable precise data transfer and safe power delivery whenever human health is in danger.
Why Medical Wire Harnesses Are Essential
Medical equipment works under zero-tolerance conditions for failure. A harness breakdown may lead to erroneous readings, disrupted treatment, or even patient harm. This is why medical wire harnesses are built to a higher standard than consumer cables.:
- Precision & Reliability: Harnesses must transmit signals and electricity uninterrupted. Even slight flaws might be life-threatening. For example, a minor break in an ECG lead harness could cause heart monitoring data to be distorted. Medical wire harnesses are 100% continuity and insulation tested to detect any flaws. They’re engineered to deliver signals accurately under all conditions.
- Strict Compliance & Safety Standards: Medical harnesses adhere to strict restrictions. Their manufacture is governed by quality management systems such as ISO 13485, and it must conform to electrical safety requirements (IEC 60601 series worldwide, UL 60601-1 in the United States). Manufacturers also follow FDA standards (CFR 21 Part 820), which include design controls and risk management for all medical devices. In other words, each medical cable or harness is manufactured using a verified procedure, tested to meet FDA/CE standards, and created from biocompatible, non-toxic materials. Without this level of conformity, cable assemblies cannot be trusted in critical care.
- Durability in Harsh Environments: Hospital equipment is cleaned, sterilized, and used intensively. Medical harnesses use materials (e.g. silicone, Teflon) that resist heat, chemicals, and wear. They withstand autoclave cycles and repeated flexing. Special coatings and strain reliefs help to prevent cracks and shorts. In brief, they are designed to withstand conditions that would cause ordinary cables to break.
- Compact & Organized Design: These assemblies save space within devices by bundling multiple wires into a single harness. This compactness lowers clutter and facilitates assembly and maintenance. A single multi-core harness replaces dozens of unsecured cables, making the equipment more durable and easier to maintain.
- Electromagnetic Shielding: Medical devices (particularly imaging and monitoring equipment) are susceptible to electrical noise. Harnesses often feature EMI/EMC shielding (braids or foil) to maintain clean signals. Proper shielding is necessary to prevent X-ray pulses or defibrillator bursts in one area of a machine from distorting signals in another.
- Customization & Biocompatibility: Every gadget is unique, and harnesses are custom-designed in every case. Manufacturers select wires, connectors, and enclosures based on the application’s particular demands – whether that’s a waterproof jacket for an implanted device or an extra-flexible cable for wearable sensors. Many harnesses are even coated with antibacterial coatings or certified for installation to fulfill healthcare-specific standards.

Medical wire harnesses are tested far more rigorously than consumer cables since they transmit life-critical information and electricity. In the plant, each assembly undergoes rigorous quality control. Automatic machines neatly cut wires and crimp or solder them. Inspectors perform 100% electrical testing (continuity, insulation, and hi-pot) as well as mechanical tests (pull force, flex fatigue). Before final assembly, we cross-check each harness against the design drawings to ensure accuracy. For example, each device, whether it’s a multi-lead ECG wire or a ventilator harness, is tested to ensure it meets criteria and can withstand the hospital environment.
In reality, this means a customer can rely on their Electrode Lead Wire Harness for Physiotherapy or Ventilator Terminal Wiring Harness Assembly arriving defect-free. Our in-house testing ensures that each cable meets ISO 13485-based quality standards, guaranteeing the gadget operates flawlessly in the clinic or operating room.
Standards and Certifications
To ensure safety and compliance, medical wire harnesses must adhere to several international standards and regulations. Common requirements include:
| Standard/Certification | Scope |
|---|---|
| ISO 13485 | Environmental standards that restrict the use of hazardous substances in materials. |
| IEC 60601 series | Global electrical safety and performance for medical equipment. |
| UL 60601-1 | U.S. electrical safety standard for medical devices. |
| FDA 21 CFR Part 820 | U.S. regulations for medical device quality systems and design control. |
| IPC/WHMA-A-620 | Industry standard for wire harness assembly acceptance (IPC/WHMA A-620). |
| REACH / RoHS | Environmental standards restricting hazardous substances in materials. |
| Biocompatibility (ISO 10993) | Safe materials for patient-contact devices. |
All medical harness makers must demonstrate compliance with these criteria. Harness materials are frequently certified for biocompatibility according to ISO 10993, ensuring safe interaction with patients. Assemblies are manufactured in ISO 13485-certified facilities. Many firms also adhere to the IPC/WHMA-A-620 assembly quality criteria and utilize UL-certified wires for the US market. Harnesses can also be designated IP-class for water and dust protection.
Frequently Asked Questions
A: Medical harnesses, unlike regular cables, are manufactured in a clean-room environment and rigorously checked for defects. They are composed of medical-grade, biocompatible materials and must adhere to stricter criteria to be sterilizable, non-toxic, and dependable under extreme conditions.
A: All harnesses are thoroughly tested electrically and mechanically, including continuity checks, insulation resistance (hi-pot) tests, pull and flex tests, and sometimes imaging (X-ray) inspection of crimps. We perform 100% continuity and high-voltage tests on all assemblies, as well as environmental tests (temperature cycling, humidity, and sterilization) to ensure long-term durability.
A: They are responsible for the safety of the patients. Faulty wiring can result in misdiagnosis or device failure. A single broken cable in a surgical robot or ICU monitor can cause the entire system to fail. Medical wire harnesses reduce this risk by employing redundant design, insulation, and strict quality control to ensure signal accuracy when it matters most.
A: Yes. Manufacturers customize each harness to meet the specific device, specifying the number of leads, connector types, shielding, length, and even color coding. Customization is standard: harnesses are made to the device’s specifications, whether it’s a tiny cable bundle for a handheld endoscope or a sophisticated multi-connector harness for an MRI system.
Conclusion
Medical wire harnesses are the unsung heroes of healthcare equipment. They keep imaging equipment, monitors, surgical tools, and home-care gadgets working smoothly by ensuring every electronic connection is safe and reliable. Because lives are at stake, these harnesses are made from the highest quality materials and undergo the most stringent testing and certification processes. Partnering with experienced, ISO-certified manufacturers ensures that medical OEMs receive harnesses that meet all requirements, delivering the precision and performance that patients and doctors need.
For dependable, thoroughly tested medical wire harness assemblies that meet or exceed international standards, rely on skilled contract manufacturers with a proven track record of excellence.
.avif)
Sam Wu is the Marketing Manager at Romtronic, holding a degree in Mechatronics. With 12 years of experience in sales within the electronic wiring harness industry, he manages marketing efforts across Europe. An expert in cable assembly, wiring harnesses, and advanced connectivity solutions, Sam simplifies complex technologies, offering clear, actionable advice to help you confidently navigate your electrical projects.


